Transaction on Biomedical Engineering Applications
and Healthcare (TBEAH)
and Healthcare (TBEAH)
PUBLICATION ETHICS AND PUBLICATION MALPRACTICE STATEMENT -
Establishing ethical standards is crucial for everyone participating in the publishing process—the author, journal editor(s), peer reviewer, and the publisher. Our journal's ethics statements draw inspiration from the Committee on Publication Ethics (COPE) Best Practice Guidelines for Journal Editors.
Manuscripts under review are assessed for their intellectual merit, with no consideration given to the authors' race, gender, sexual orientation, religious beliefs, ethnic background, citizenship, or political stance.
The Editor-in-Chief and editorial staff are obligated to maintain the confidentiality of submitted manuscripts. Any information about a manuscript should only be disclosed to the corresponding author, reviewers, potential reviewers, other editorial advisers, and the publisher, as necessary.
Any unpublished material revealed in a submitted manuscript cannot be utilized in an editor's own research without explicit written consent from the author(s).
The Editor-in-Chief of the journal is tasked with determining which submitted articles should be published. This decision is influenced by the journal's Editorial Board policies and is subject to legal requirements such as those concerning libel, copyright infringement, and plagiarism. Collaboration with other editors or reviewers may occur in reaching this decision.
Peer review plays a vital role in aiding the Editor-in-Chief in making informed editorial decisions. Additionally, it serves as a means of editorial communication with the author, facilitating potential improvements to the manuscript.
If an invited referee feels inadequately qualified to review the manuscript or anticipates a delay in the review process, they should promptly inform the Editor-in-Chief. This allows for the identification of alternative reviewers.
Manuscripts designated for review are to be treated as confidential documents. Revealing or discussing them with unauthorized individuals is strictly prohibited unless explicitly authorized by the Editor-in-Chief.
Reviews should be conducted objectively, with a focus on the content rather than personal criticism of the author. Referees are expected to express their views clearly, supported by appropriate arguments..
Reviewers are responsible for identifying relevant published work that the authors have not cited. Any mention of prior reporting of observations, derivations, or arguments should be accompanied by the appropriate citation. Additionally, reviewers should bring to the Editor's attention any significant similarity or overlap between the manuscript under consideration and their personal knowledge of other published data.
Information or ideas obtained through peer review are confidential and should not be used for personal gain. Reviewers are expected to refrain from evaluating manuscripts in which they have conflicts of interest arising from competitive, collaborative, or other relationships with the authors, companies, or institutions associated with the submission.
Authors presenting the findings of original research are obligated to provide an accurate account of the conducted work and an impartial discussion of its significance. The manuscript should faithfully represent the underlying data, offering sufficient details and references to enable replication of the study by others. Engaging in fraudulent or knowingly inaccurate statements is considered unethical and unacceptable.
Authors must ensure that their works are entirely original. If they incorporate the work or words of others, proper citation or quotation should be employed to acknowledge the sources.
Authors should refrain from publishing manuscripts describing essentially the same research in more than one journal. Simultaneous submission of the same manuscript to multiple journals is deemed unethical publishing behavior and is not acceptable.
Authorship should be confined to individuals who have significantly contributed to the conception, design, execution, or interpretation of the reported study. All substantial contributors should be listed as coauthors, while those involved in specific substantive aspects should be acknowledged. The corresponding author holds the responsibility to ensure that the author list includes appropriate contributors, that no inappropriate co-authors are included, and that all co-authors have reviewed and approved the final version of the paper and agreed to its submission for publication.
If the research involves hazardous chemicals, procedures, or equipment, authors must clearly identify these in the manuscript.
Authors are required to disclose any financial or substantive conflict of interest in their manuscript that could potentially influence the results or their interpretation. All sources of financial support for the project should be disclosed.
In the event of a significant error or inaccuracy discovered by an author in their published work, it is their duty to promptly inform the Editor-in-Chief or publisher of the journal and collaborate to either retract the paper or publish an appropriate erratum.
In instances of suspected or proven scientific misconduct, fraudulent publication, or plagiarism, the publisher will work closely with the Editors-in-Chief to take necessary actions for clarification and correction of the situation. This may involve promptly publishing an erratum or, in severe cases, retracting the affected work entirely.
The Publisher and the Journal adhere to a non-discriminatory policy in their publishing programs, services, and activities. Regardless of age, color, religion, creed, disability, marital status, veteran status, national origin, race, gender, genetic predisposition or carrier status, or sexual orientation, equal treatment is maintained.
LICENCES, COPYRIGHT & PERMISSIONS
All the articles published in the journal of TBEAH is licensed with Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
As a user, you have the right to request to publish an article and make it available, we need publishing rights from you for that work. We therefore ask authors for publishing in Journal of Pharmaceutical Research and Innovation to sign an author contract which grants us the necessary publishing rights. This will be after your manuscript has been through the peer-review process, been accepted and moves into publishing. Our Publication team will then send you an email with all the details.
To protect the rights and interests of both parties, Journal of Pharmaceutical Research and Innovation requires an exclusive licence that clearly stipulates our rights and the specific rights retained by authors. We ask the corresponding author to grant this exclusive licence to Journal of Pharmaceutical Research and Innovation on behalf of all authors. Journal of Pharmaceutical Research and Innovation agrees to publish the manuscript and has the right:
In our standard author contract, you transfer – or "assign" – copyright to us as the owner and publisher of the journal .
Authors are allowed to use their own articles for non-commercial purposes without seeking permission from Transaction on Biomedical Engineering Applications, Healthcare. For commercial use we need to know about it. Authors retain the following right to:
Post a PDF of their article to fellow researchers for educational purposes only;
Make a reasonable number of copies for personal or non-commercial use. This includes the contributor's own teaching purposes;
Republish part or all of the article in a book or other publication edited by the author (except for multiple contributions in the same book or publication);
Use the illustrations and research data in their own future works;
Use the article in course packs in the authors institution. This does not apply if a commercial charge is made for the compilation or training programme;
Use the work for further research and presentations at meetings;
to authorize Transaction on Biomedical Engineering Applications, Healthcare to take legal proceedings in the copyright owner's name and on the authors's name. Reuse all or part of the published manuscript in other works for non-commercial purposes, the original publication in the Journal of Pharmaceutical Research and Innovation must be approved through a note or citation in a format acceptable to Transaction on Biomedical Engineering Applications, Healthcare.
OPEN ACCESS ARTICLES
Transaction on Biomedical Engineering Applications, Healthcare is committed to real and immediate open access for published articles. All of the TBEAH articles are free to access immediately from the date of publication. There are no charge for any reader to download articles
Information about all funding sources pertaining to the conducted work should be presented in a distinct section titled 'Funding.' This section should follow the 'Acknowledgements' part.
The following guidelines are to be adhered to:
The sentence should commence with: 'This work was supported by ...'
The complete official name of the funding agency should be provided, such as 'Technoarete Research and Development Association,' not abbreviated as 'TRDA.'
Agencies should be delineated by a semi-colon, with 'and' preceding the last funding agency.
Any specific role played by a sponsor should be explicitly outlined in the acknowledgements section. This includes their involvement in writing, study design, data collection, analysis, or interpretation.
In the abstract, if funding has been secured for the study, a 'Funding:' heading should be added at the end, enumerating the companies or institutes that have provided financial support. For instance, Funding: ARC.
CONFLICTS OF INTEREST POLICY
Authors are obligated to disclose any actual or potential conflict of interest, encompassing financial,
personal, or other relationships that could unduly influence or be perceived to influence their work.
This disclosure is required at the time of manuscript submission and is reiterated during the
submission of the Author Agreement Form. The second declaration in the Author Agreement Form
serves two purposes:
i) authors can rectify any inadvertent omissions in the initial conflict of interest
declaration made during manuscript submission, and
ii) if any conflicts arise between manuscript
submission and the final decision, authors can declare them in the agreement for appropriate action.
The corresponding author bears the responsibility of gathering potential conflicts of interest from each author and incorporating the comprehensive list into the manuscript before submitting it for publication.
Editors of all journals published by TBEAH are committed to upholding the highest standards in manuscript evaluation and preserving the integrity of the Journal. Editors must disclose any potential conflicts of interest to TBEAH Journals at the time of enrollment, as well as promptly upon the development of any conflicts after enrollment. This disclosure is also required separately for each manuscript undergoing editorial review. If an editor submits a manuscript for publication, the handling editor's identity will be known only to the Editor-in-Chief and the Journal editorial staff.
When a manuscript is submitted by an author from the same institution as one of the editors, it will be assigned to another editor not affiliated with that institution. Similarly, if an author has a familial, personal, or professional relationship with an editor, the manuscript will be assigned to a different editor.
All peer reviewers must declare any conflicts of interest in the manuscript review form as the initial step in the manuscript evaluation process. If a material potential conflict, whether financial or otherwise, exists, reviewers are encouraged to recuse themselves from the review process. They should submit the review form to the Editor-in-Chief, listing the conflict of interest. Importantly, the reviewer's declaration of a potential conflict does not automatically invalidate the review of the manuscript.
EDITORIAL POLICIES
Author(s) should avoid the research and publication misconduct. If some cases of research and publication misconduct occur within each step of submission, review, edition or publication, journals have the right to legal action. The cases are listed as below:
Reviewers must consider the followings:
Reviewers must consider the followings:
REVIEWERS & REVIEW PROCESS
Reviewers evaluate article submissions to journals based on the requirements of that journal, predefined criteria, and the quality, completeness and accuracy of the research presented. They provide feedback on the paper, suggest improvements and make a recommendation to the editor about whether to accept, reject or request changes to the article. The ultimate decision always rests with the editor but reviewers play a significant role in determining the outcome.
PEER REVIEW PROCESS
Manuscript Review
Submitted manuscripts are first screened by the editorial staff for completeness and to determine if
the manuscript meets the general criteria for the journal. The Editor will decide to:
(a) send the
manuscript out for double blind review,
(b) request initial revisions prior to the double blind review
process, or
(c) reject the manuscript.
Each manuscript passing initial plagiarism will be subjected to rigorous and anonymous peer-review by a minimum of 2 peer reviewers. Referees who review a manuscript remain unknown to the authors. The journal’s independent status ensures a submission acceptance rate based on merit and not favor, bias, or personal preference. All the reviewers send the editor a detailed report with their comments on the manuscript and their recommendation. Authors receive reviewers’ recommendation by the editorial staff and they never enter in contact with reviewers. Reviewers have to complete their reviews within 2-3 weeks. For papers which require revision, the editor will make sure that the quality of the revised paper is acceptable.
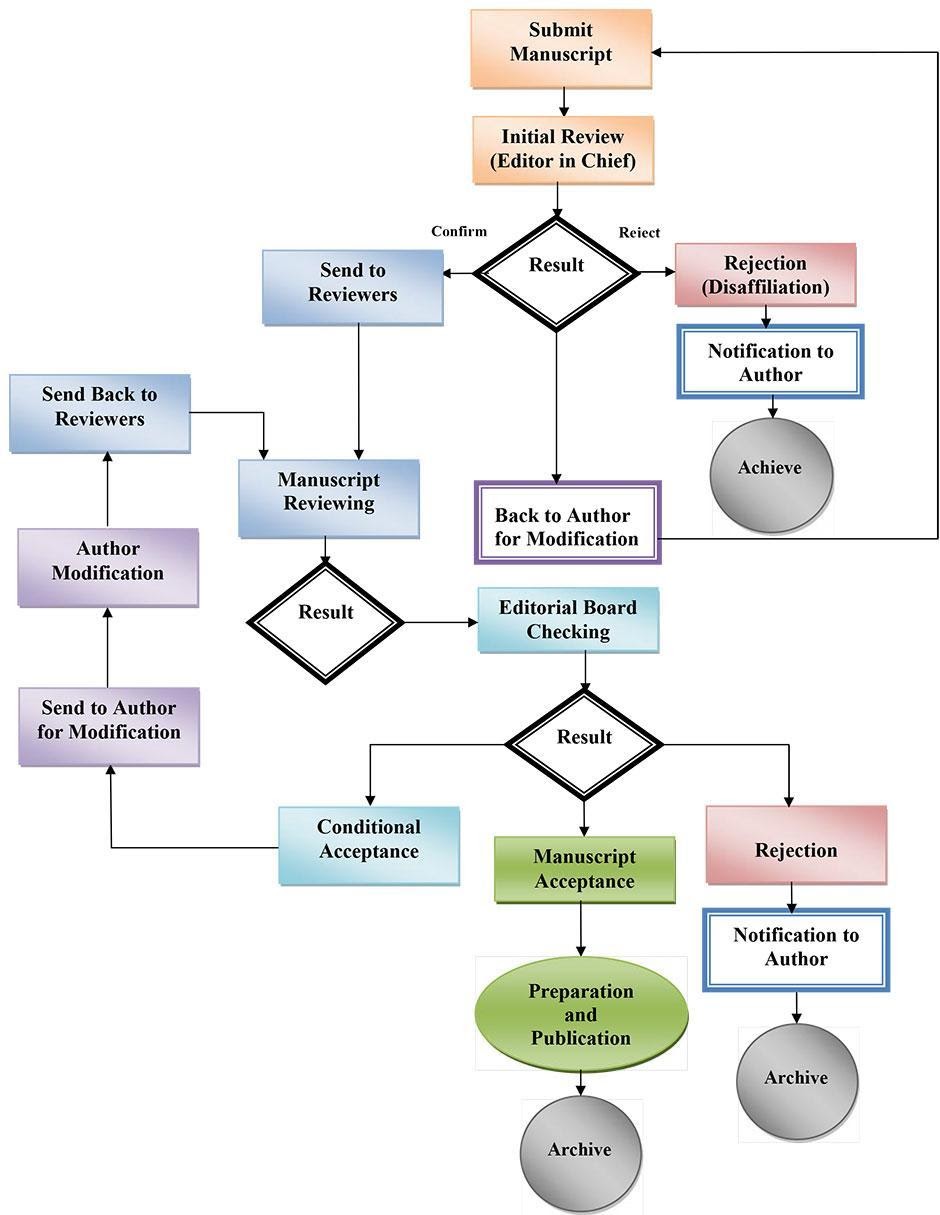
PEER REVIEW / RESPONSIBILITY FOR THE REVIEWERS
Peer review assists the Editor in Chief and the editorial board in making editorial decisions while editorial communications with the author may also assist the author in improving the paper.
Any selected referee who feels unqualified to review the assigned manuscript or unable to provide a prompt review should notify the editor and excuse himself from the review process.
Manuscripts received for review must be treated as confidential documents. They must not be shown to, or discussed with, others except as authorized by the Editor-in-Chief.
Reviews should be conducted objectively. There shall be no personal criticism of the author.
Reviewers should express their views clearly with supporting arguments.
Reviewers should identify relevant published work that has not been cited by the authors. Any statement that had been previously reported elsewhere should be accompanied by the relevant citation. A reviewer should also call to the Editor-in-Chief's attention any substantial similarity or overlap between the manuscript under consideration and any other published paper of which they have personal knowledge.
Privileged information or ideas obtained through peer review must be kept confidential and not used for personal advantage.
Reviewers should not review manuscripts in which they have conflicts of interest resulting from competitive, collaborative, or other relationships or connections with any of the authors, companies, or institutions connected to the papers.
ADVERTISING POLICY
TBEAH Journal does not publish any advertising or sponsorship opportunity, as a means to provide high value to our readers.
OPEN ACCESS
Transaction on Biomedical Engineering Applications, Healthcare is committed to real and immediate open access for published articles. All of the TBEAH articles are free to access immediately from the date of publication. There are no charge for any reader to download articles.
ROLE OF THE CORRESPONDING AUTHOR
One author is assigned as Corresponding Author and acts on behalf of all co-authors and ensures that questions related to the accuracy or integrity of any part of the work are appropriately addressed
The Corresponding Author is responsible for the following requirements:
Please check the Instructions for Authors of the Journal that you are submitting to for specific instructions regarding contribution statements.In absence of specific instructions and in research fields where it is possible to describe discrete efforts, the journal recommends authors to include contribution statements in the work that specifies the contribution of every author in order to promote transparency. These contributions should be listed at the end of the submission.
*The requirement of managing all communication between the journal and all co-authors during submission and proofing may be delegated to a Contact or Submitting Author. In this case please make sure the Corresponding Author is clearly indicated in the manuscript.
PUBLICATION FREQUENCY
Transaction on Biomedical Engineering Applications, Healthcare (TBEAH) is a bi-annual journal and publishes new issues twice every year, during the months of January & July.